Biolab > Training > Technology
Spectrophotometers
Absorption spectrophotometry can be used to measure the; 1) concentration of a substance in solution, 2) properties of many types of molecules, and 3) biological activities of living cells, such as enzyme activities and rates of photosynthesis. When studying a compound in solution by spectrophotometry, you put it in a sample holder called a cuvette (Figure 1A) and place it in the spectrophotometer. Light of a particular wavelength passes through the solution inside the cuvette and the amount of light transmitted (Transmittance) or absorbed (Absorbance) by the solution is measured by a light meter. The light that passes through the sample (not absorbed) is called transmitted light. This difference in the original and transmitted light is called absorbance. While a spectrophotometer can display measurements as either transmittance or absorbance, in biological applications we are usually interested in the absorbance of a given sample. Because other compounds in a solution (or the solvent itself) may absorb the same wavelengths as the compound being analyzed, we compare the absorbance of our test solution to a reference blank. Ideally, the reference blank should contain everything found in the sample solution except the substance you are trying to analyze or measure. Spectrophotometers are designed to transmit light of narrow wavelength ranges (Figure 1B). A given compound will not absorb all wavelengths equally which is why we see things as different colors (some compounds absorb only wavelengths outside of the visible light spectrum, and that’s why there are colorless solutions like water). Since different compounds absorb light at different wavelengths, a spectrophotometer can be used to distinguish compounds by analyzing the pattern of wavelengths absorbed by a given sample. Additionally, the amount of light absorbed is directly proportional to the concentration of absorbing compounds in that sample, so a spectrophotometer can also be used to determine concentrations of compounds in solution. Finally, because particles in suspension will scatter light (thus preventing it from reaching the light detector), spectrophotometers may also be used to estimate the number of cells in suspension.
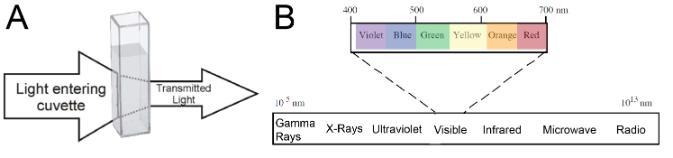
Figure 1. A) Absorption of light as it passes through a solution and B) the electromagnetic spectrum. Visible light (400-700 nm) constitutes only a small portion of the spectrum.
Directions
Remove the plastic protective cover and turn on the spectrophotometer using the power switch located on the back of the machine, lower left side. The spectrophotometer will automatically perform some diagnostics and will need to warm up for 30 minutes before measurements can be made. Once it has warmed up, it should automatically be set to absorbance (A) mode. If it is not, press the A/T/C button to select absorbance (A).
- Measure your blank by adding a blank solution to the cuvette (or test tube). Wipe down the outside of the test tube with ethanol and a kimwipe to remove fingerprints.
- Insert your cuvette into the cell holder of the sample chamber. The cuvette should be sitting on the bottom of the cell.
- Close the sample chamber.
- Press the ABS button to set the blank to 0 concentration.
- Select the wavelength you will be measuring your solutions at by press the nm buttons.
- Measure your samples by inserting the cuvette into the cell holder. Remember to clean the outside of the cuvette before placing it into the cell holder. Do not get fingerprints on the cuvette as it will disrupt the reading.
- Read your absorbance.
- Remove your sample replace it with a new sample and record the absorbance.
At the end of the lab period (if there isn’t a lab immediately following), shut off the machine and replace the plastic cover.
If you receive an error message use the Operator's Manual for Genesys 20 Spectrophotometer.
