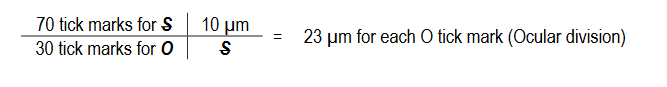Biolab > Training > Technology
Microscopy
Resolution | Stereo | Compound | Köhler Illumination | Calibration | Leica Camera
Magnification is the process of enlarging the apparent size, not the physical size, of something. In microscopy, it is the ratio between the size of an image produced by the microscope and its actual size. Microscopes magnify thin specimens mounted on microscope slides. They are ideal for observing unicellular or very small organisms, cells, and cell structures. We will use the compound and dissecting microscopes many times over the course of the semester. It is important to familiarize yourself with microscope use.
Resolution
The resolving power of a microscope is dependent on the numerical apertures of the optical lenses and the wavelength of light used to examine the specimen. It is the smallest distance between two points (measured in microns) that can be seen with the microscope. If two small objects close together can be seen clearly as two distinct objects, a microscope is said to have high resolving power.
Lateral Resolution: point-to-point resolving power in the plane perpendicular to the optical axis. It is usually defined as the shortest distance between two lateral points on the specimen plane that can still be distinguished as separate entities.
Axial Resolution: point-to-point resolving power in the plane parallel to the optical axis. It is usually defined as the shortest distance between two longitudinal points on the specimen plane that can still be distinguished as separate entities.
Depth of Field: is determined by the distance from the nearest specimen plane in focus to that of the farthest plane also simultaneously in focus. The thickness of the optical section along the optical axis within which objects in the specimen plane are in focus. High-magnification objectives have a decreased depth of field. The reverse is true of low-magnification objectives
Field of View: the visible area seen through the microscope when the specimen is in focus. The greater the magnification the smaller the view.
Focus: a specimen is in focus at the desired magnification when the image seen through the ocular lens is sharp and clear.
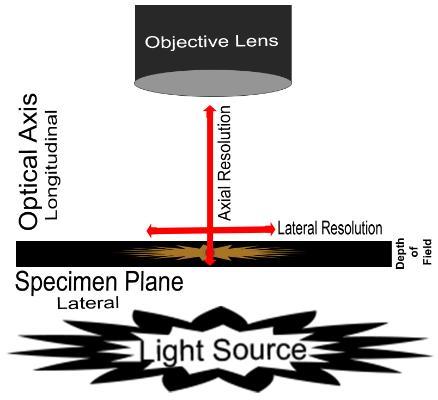
Figure 1. Diagram demonstrating resolution and depth of field.
Stereo Microscopes
Stereo microscopes have low magnifications that can range from 2 to 100x depending on the microscope and are designed for viewing whole objects like rocks, plants, flowers, and invertebrate organisms by reflecting light off the specimen, producing a 3-dimensional image. Sometimes there is a light located in the base of the microscope that will allow transmitted light.
Stereo Head: the structure that the ocular eyepieces are attached to.
Ocular lens or eyepiece: the part of the microscope that you look through.
Diopter: compensates for focusing differences between your eyes, it is very important this is set correctly, to prevent eye strain.
Zoom Magnification knob: zoom magnification enables you to zoom through a magnification range.
Focusing knob: the knob that allows you to focus on the object at each magnification by moving the stereo head up or down.
Illuminator or light source: the light source can be built into the base of the microscope, transmitting light through the specimen, and/or the light source may be above the specimen as incident light. The lights can be turned on using a rheostat (light) control knob on the front of the base.
Arm (Pillar): the frame that supports all components above the base.
Base: the bottom of the microscope, which supports the entire instrument. The stage plate is located directly on the base surface upon which a specimen is placed. The stage can have a removable black or white tile (that can be removed and cleaned) or it will have a light that will transmit light through the specimen.
Setting Up
- Always carry the microscope upright with both hands - one hand grasping the arm of the microscope and the other hand supporting its base.
- Place the microscope on your lab bench, away from the edge, with the microscope arm facing away from you.
- Remove the dust cover, fold it, and put it aside.
- Plug in the microscope. Make sure that the cord is positioned where it will not get in the way.
- Turn on the light source in the base. Adjust the illumination for your comfort.
Putting Away
- Remove the specimen from the stage.
- Turn off the light and allow it to cool.
- The lowest power objective should be in place for microscope storage.
- Make sure that the stage is clean.
- Wrap the electrical cord around the ocular NOT the arm.
- Replace the dust cover.
- Return the microscope to the cabinet or cart you originally found it in.
C. Compound Microscopes
A compound microscope is a high-power microscope that uses a compound lens system. Higher magnification is achieved by using two lenses rather than just a single magnifying lens. While the eyepieces and the objective lenses create high magnification, a condenser beneath the stage focuses the light directly into the sample. A compound microscope has multiple lenses: the objective lens (typically 4x, 10x, 40x, or 100x) is compounded (multiplied) by the eyepiece lens (typically 10x) to obtain a high magnification of 40x, 100x, 400x, and 1000x. The objective lenses of a compound microscope cause the orientation of the image of the specimen to be inverted compared to the orientation of the actual specimen which means that a specimen viewed through a compound microscope will look upside down and backward compared to how the specimen is mounted on the slide.
Base: the bottom of the microscope, which supports the entire instrument.
Condenser: the lens located below the stage, which focuses light (from the illuminator) through the specimen being observed. Most microscopes have a movable condenser allowing its distance from the specimen to be adjusted using the condenser knob and condenser alignment screws.
Illuminator or light source: the light source is usually built into the base of the microscope, and directs light through the condenser to the specimen. Alternatively, the light source may be separate, and be directed toward the condenser with a mirror. The intensity of the light can be adjusted using the rheostat (light) control knob. The microscope you are using has a rheostat on the front of the base and a switch on the left of the base.
Arm: the frame that supports all components above the base.
Revolving nosepiece or turret: a revolving disc-shaped support or frame for the objective lenses.
Stage: the flat surface upon which the slide with your specimen is placed. Most microscopes have a stage finger assembly to hold the slide on the stage. The entire mechanism including the slide moves horizontally across the stationary stage (left/right and forward/back) using two-stage adjustment knobs situated under the stage (variably on the left or right side, in front of the focusing knobs).
Iris diaphragm: a unit below the condenser that controls the amount of light directed to the specimen. The diameter of the diaphragm can be adjusted by turning it to increase or decrease the size of the hole that light passes through.
Coarse adjustment or coarse focusing knob: the large knob towards the back of the instrument that is used to significantly raise or lower the stage, when you first focus on a specimen at low power. It is never used when high-power objectives are in place.
Fine adjustment or fine focusing knob: the smaller knob towards the back of the instrument that is used to make small adjustments in the height of the stage for final focusing on a specimen. It is the only focusing knob used with high-power objectives.
Ocular lens or eyepiece: the secondary optical system that you look through. The ocular lens further magnifies (10x) the image and brings the light rays to a focal point. A binocular microscope has two ocular lenses and a monocular microscope has one ocular lens that sits on the adjustable binocular body. Binocular lenses can be adjusted to fit the distance between your eyes by gently pulling the oculars apart or by pushing them closer together.
Objective lenses: the primary optical system which produces a magnified image of the specimen. There are typically four objective lenses attached to the nosepiece with the magnification of each objective engraved on its side.
- 4X scanning
- 10x low power
- 40X high power
- 100X oil immersion
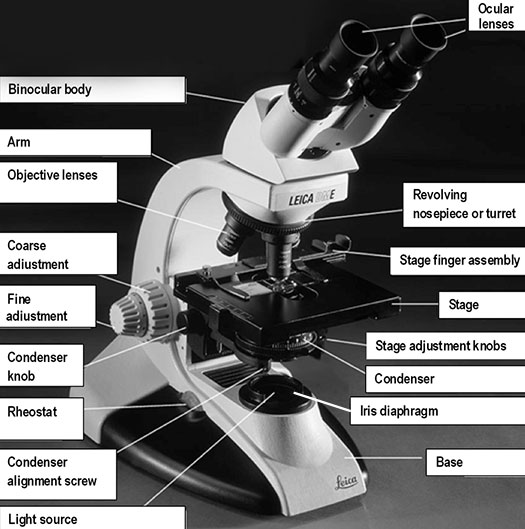
Figure 2. Diagram demonstrating the parts of a compound microscope.
The 100X objective lens is called an oil immersion lens because oil is placed between the lens and the microscope slide to increase resolution (i.e., the level of detail that can be observed in an image). Light bends when it passes from the glass slide to air because of differing refractive indices. A drop of immersion oil between the slide and lens eliminates this problem because the oil has the same refractive index as the glass slide. Never use the 100X objective lens without oil and do not get oil on the 4X, 10X, or 40X lenses.
- Focus very carefully with the 40x objective over the stained specimen on the slide (once focused, do not alter focus for the next three steps.)
- Rotate the turret halfway so that the 40x and 100x objectives straddle the specimen.
- Never get oil on any other objective lens.
- Apply a small drop of oil directly on the slide over the specimen.
- Rotate 100x objective into the immersion oil.
- When finished, clean the oil from the lens using lens paper and cleaner.
- Always carry the microscope upright with both hands - one hand grasping the arm of the microscope and the other hand supporting its base.
- Place the microscope on your lab bench, away from the edge, with the microscope arm facing away from you.
- Remove the dust cover, fold it, and put it aside.
- The compound microscope is usually stored in its storage position, with the eyepieces pointed backward toward the arm. This is not the position with which it is to be used. Gently rotate the entire binocular head into its working position. This may require that you temporarily loosen (and then retighten) a screw holding the head onto the microscope.
- Plug in the microscope. Make sure that the cord is positioned where it will not get in the way.
- Adjust the nose piece so that the scanning lens (4X) is pointing directly toward the stage. To prevent the objective lenses from being damaged before you place a slide in the slide holder on the stage, you must separate the objectives from the stage as much as possible. Depending on the design of the microscope, you will do this by using the coarse objective knob to move the stage down or the nosepiece up (i.e., away from the objective).
- Turn on the light source in the base. Adjust the illumination for your comfort.
- Adjust the distance between the ocular lenses for your comfort.
- Once you are done using the microscope:
- turn off the light,
- clean the lenses (eyepieces and objectives) with lens paper and cleaner,
- clean the stage (slide put away),
- 4x objective down towards the stage,
- stage at the lowest point,
- unplug it, wrap the cord around the ocular lenses, and put on the dust cover.
- Add a drop of suspension to the center of a slide.
- Place a cover slip on the slide at an angle so that it touches the drop.
- Slowly lower the raised end of the cover slip so that it forces the drop to flow away from the edge of the coverslip that is touching the slide.
- Place the wet mount on the stage of the microscope.
- Do not use the 100X objective.
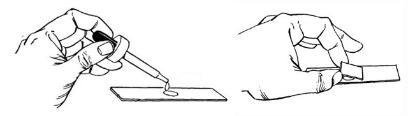
Figure 3. Diagram demonstrating how to make a wet mount.
Köhler Illumination
Köhler illumination is the alignment of the image-forming light path and the illumination light path of the microscope. In this process the condenser is centered and focused, thereby providing an evenly illuminated field of view and more importantly maximum resolution of the specimen
- Position the focusing eyepiece to the appropriate number for your eyesight on the left eye-piece.
- Position a stained slide on the stage.
- Focus the specimen using the 10x objective and comfortable illumination intensity, adjusting the focusing eyepiece as needed.
- Close the field diaphragm (black lever below the stage and condenser) until about ¼ of the field of view is illuminated.
- Partially close the aperture diaphragm (on the condenser).
- Use the condenser height adjustment knobs to position the condenser so the diaphragm image is in focus.
- Use the two condenser centration screws to position the condenser in the diaphragm image in the center of the field of view.
- Fully open the field diaphragm.
- Adjust the aperture diaphragm for appropriate contrast (also dependent upon the intensity of the stain and thinness of the sample).
- Your microscope is now set to maximize the resolution of the specimen.
Note: The microscope is now set to maximize the resolution of the specimen. If you adjust the condenser height to gain contrast or adjust light intensity you will sacrifice the resolution capability. Use the aperture diaphragm and /or the illumination intensity to adjust contrast.
Calibration
Microscopes must be calibrated so accurate measurements can be made. To calibrate a microscope both an ocular and a stage micrometer are used.
- The ruler’s magnification does not change when switching between the objective lenses.
- At each objective, the value (in μm) of an ocular tick mark (division) must be determined.
- The ruler’s magnification does change when switching between the objective lenses.
- The ‘ruler’ is usually 1 to 2 mm (1000 to 2000 μm) across.
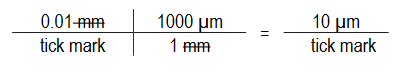
- Each tick mark (divisions) on the ruler is 10μm (0.01mm) from the next tick mark.
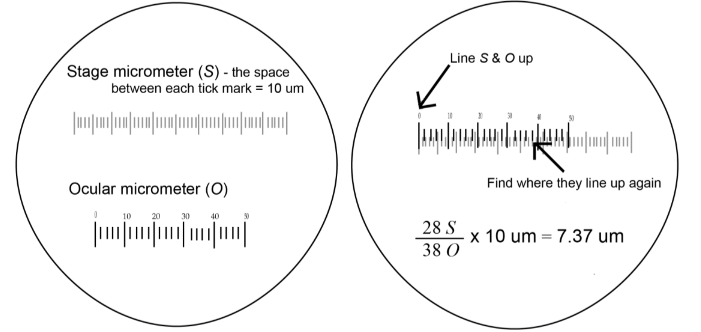
- Focus on the stage micrometer (S).
- Rotate the ocular lens until the ocular micrometer (O) is exactly superimposed on S.
- Adjust S so that its first tick mark lines up precisely with O’s first tick mark.
- Look for another tick mark (as far from the beginning as possible) where S and O line up precisely.
- Count the number of tick marks between these two tick marks for both O and S.
Example 1:
Example 2:
Leica Application Suite (LAS EZ)
Microscopes are used by the students in many lab exercises. Instructors also need to learn to use the instructor microscope with the Leica camera and required LAS EZ & Leica AirLab Icon Guide software which will allow them to project the microscope images in real time.
A. Instructor Microscope:
- Plug the microscope into a power outlet.
- Turn on the microscope using the green toggle switch at the base of the microscope.
- Focus a slide on the microscope.
B. Camera on the Instructor Microscope:
- Make sure the camera is connected via USB to the computer.
- Turn on the camera by pressing the red button, the camera icon, and the USB icon (all on the right side of the camera.)
C. LAS EZ Software:
- Using Windows search, search for LAS EZ.
- Open the program; it may take a minute or two for the software to connect to the camera.
- In the upper right area of your screen of the LAS EZ software you will see three tabs: 'Acquire', 'Browse', & 'Process'.
- Select the ‘Acquire’ tab to project or take a photo.
- Save the image by clicking on the 'Acquire Image' button or record a video of a live specimen by clicking on the video camera icon under 'Image Controls'.
- Once you click on the 'Process' tab you can measure and annotate the images you take of your slide specimens.
- Once you have saved it, open the 'Browse tab'. You will need to move a copy either to the C: drive or to the lab Google Drive
D. Projector:
- Turn on the projector.
- Focus the slide using the projected image.
- Adjust the brightness and contrast of the projected image using the software.